Background:Murine double minute 2 (MDM2), a key negative regulator of the tumor suppressor protein p53, is overexpressed in malignancies such as MF and Merkel cell carcinoma (MCC). Navtemadlin is a potent, selective, orally available MDM2 inhibitor that overcomes MDM2 dysregulation by restoring p53 activity and inducing apoptosis of TP53WT tumors. Navtemadlin has been tested in 21- or 28-day treatment cycles, with 5 or 7 consecutive days of dosing per cycle.
Navtemadlin elicits plasma concentration-dependent increases in serum macrophage inhibitory cytokine-1 (MIC-1). An increase in MIC-1 relative to baseline MIC-1 serum concentration is a key pharmacodynamic (PD) marker of response to navtemadlin treatment (Zhang et al. Xenobiotica 2022).
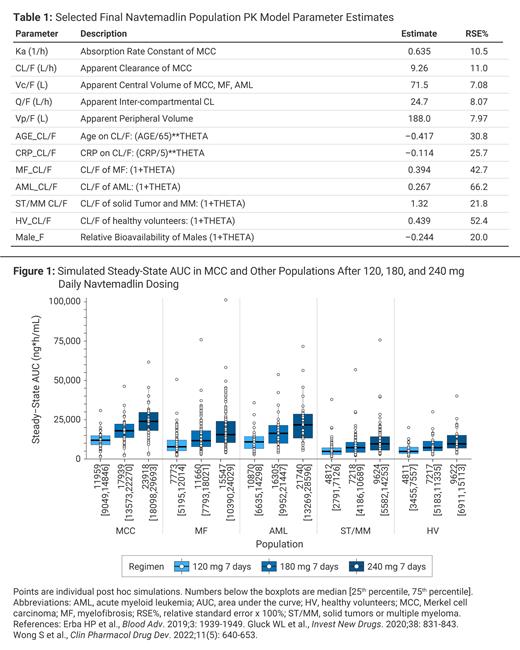
A population pharmacokinetic (PK) model was developed, based on patients with R/R MF (N=113, NCT03662126); MCC (N=35, NCT03787602); solid tumors and multiple myeloma (ST/MM N=105, Gluck et al. Invest New Drugs 2019); R/R acute myeloid leukemia (AML, N=35, Erba et al. Blood Adv 2019); and healthy volunteers (HV, N=30, Wong et al. Clin Pharmacol Drug Dev 2022).The objectives wereto characterize navtemadlin PK and covariate effects and compare steady-state (SS) PK and PD across cancer types.
Methods:A two-compartment PK model with first-order absorption and elimination was estimated using NONMEM ® (first-order conditional estimation with interaction). Four transit compartments captured absorption. Inter-subject variability was modeled as lognormal. A stepwise covariate search used forward addition (p<0.05) followed by backward elimination (p<0.01). Log-transformed SS MIC-1 ratio to baseline (fold ratio) was tested in a fixed-effects linear regression model with respect to SS exposures, with baseline MIC-1 as the covariate. The final population PK and MIC-1 models were evaluated by inspection of parameter tables, by overlaying model predictions with observed data, and by standard residual diagnostics.
Results:The PK estimation used 5,282 concentrations from 318 subjects, including 1,415 concentrations in 113 patients with R/R MF. Tumor type affected absorption rate (median, 0.635/hr in MCC; slower vs other types), apparent clearance (median, 8.90 L/hr in MCC; lower vs other types), and apparent central volume (median, 64.4 L in MCC; similar to AML, but lower vs other types) (Table 1). Accordingly, simulated SS area under the curve (AUCss) differed across populations in the order MCC>AML>MF>ST/MM = HV (Figure 1).
Covariate analysis indicated median AUC increased with the inflammation marker C-reactive protein, by 21% at 90 th percentile CRP, and with age, by 5% at 90 th percentile age of 75 y, compared to the median, 66 y. Men had 24% lower relative bioavailability than women. Taking navtemadlin with food slowed absorption by 10% but had no impact on AUCss. Other tested covariates, including body weight, creatinine clearance, aspartate transaminase, alanine transaminase, bilirubin, albumin, alpha 1-acid glycoprotein, race, UGT1A1 genotype, recent ruxolitinib treatment in MF, and CYP3A4 inducer/inhibitor concomitant medications, were not significant.
Median MIC-1 fold ratio increased with navtemadlin AUCss. Higher baseline MIC-1 resulted in lower SS MIC-1 fold ratio. Median baseline MIC-1 differed by tumor type. In R/R MF, baseline MIC-1 was 2,297 ng/L and SS fold ratio of MIC-1 was 7.79 (N=91). In MCC, baseline MIC-1 was 1,718 ng/L and SS fold ratio of MIC-1 was 16.7 (N=29). In R/R MF, higher baseline spleen volume was also associated with lower SS MIC-1 fold ratio. Exposure-MIC-1 relationships in R/R MF and MCC were generally similar to other tumor types and HV populations (Allard et al. Hemasphere 2019; Zhang et al. Xenobiotica 2022).
Conclusions:Tumor type influenced navtemadlin exposure with simulated exposure in R/R MF patients at the selected Phase 3 dose of 240 mg comparable to exposures in MCC patients at 180 mg, the selected Phase 2 dose for that indication. Cancer-associated inflammation and/or treatment history, as well as age and female sex, may increase navtemadlin exposure. The PD marker MIC-1 responds to navtemadlin in a concentration-dependent fashion. The overall SS fold change of MIC-1 PD responses was lower in disease states or subjects with higher baseline MIC-1. These studies demonstrate potent, dose-dependent PK/PD responses following p53 activation with the novel MDM2 inhibitor, navtemadlin, across diverse tumor types.
Disclosures
Zhang:Certara Inc.: Ended employment in the past 24 months; Kartos Therapeutics: Consultancy. Poland:Certara Inc.: Current Employment; Kartos Therapeutics: Consultancy. Xu:Certara Inc.: Current Employment; Kartos Therapeutics: Consultancy. Allard:Kartos Therapeutics: Current equity holder in private company; Telios Pharma: Current Employment, Current equity holder in private company. Krejsa:AstraZeneca: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Kartos Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Seattle Genetics: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Acerta Pharma: Current equity holder in private company, Ended employment in the past 24 months. Slatter:Acerta Pharma: Current equity holder in private company; AstraZeneca: Current equity holder in publicly-traded company; Kartos Therapeutics: Current Employment, Current equity holder in private company; Telios Pharma: Current equity holder in private company; Amgen: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal